
Chemistry, 31.03.2020 14:40 ashley232323
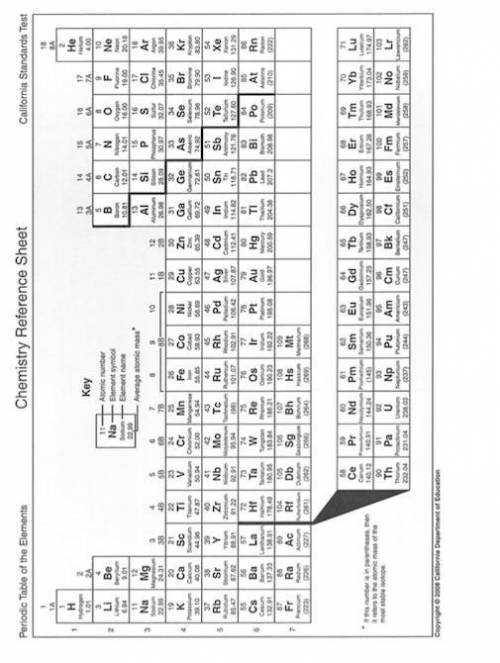
Solve the below problems relating to the molality of NaNO3 in aqueous solutions. Make use of the Periodic Table on page 2 to calculate the molar masses.
(i) A solution is prepared using 17 g of water and 1 g of NaNO3. What is the molality of the NaNO3 solution?
(ii) In order to prepare a solution of 2 molal NaNO3 using 30 g of water,
what mass of NaNO3 is required?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, Jana1517
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes. which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature. sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Solve the below problems relating to the molality of NaNO3 in aqueous solutions. Make use of the Per...
Questions in other subjects:


Mathematics, 24.09.2020 20:01



Chemistry, 24.09.2020 20:01

Mathematics, 24.09.2020 20:01


Mathematics, 24.09.2020 20:01


Mathematics, 24.09.2020 20:01



