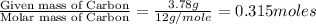Chemistry, 31.03.2020 04:06 SisterMina
Ibuprofen, the active ingredient in Advil, is made up of carbon, hydrogen and oxygen atoms. When a sample of ibuprofen, weighing 5.000 g, burns in oxygen, 13.86 g of CO2 and 3.926 g of H2O are obtained. What is the simplest formula of ibuprofen?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
Ibuprofen, the active ingredient in Advil, is made up of carbon, hydrogen and oxygen atoms. When a s...
Questions in other subjects:



English, 18.05.2021 09:00

Mathematics, 18.05.2021 09:00

Law, 18.05.2021 09:00

History, 18.05.2021 09:00

Physics, 18.05.2021 09:00







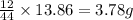 of carbon will be contained.
of carbon will be contained. of hydrogen will be contained.
of hydrogen will be contained.