
Chemistry, 31.03.2020 03:21 cathydaves
The compound carbon suboxide, C3O2, is a gas at room temperature. Use the data supplied to calculate the heat of formation of carbon suboxide. (Data: 2CO(g) + C(s) → C3O2(g) ΔH° = 127.3 kJ/mol and: ΔHf° of CO(g) = –110.5 kJ/mol)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
The compound carbon suboxide, C3O2, is a gas at room temperature. Use the data supplied to calculate...
Questions in other subjects:

Mathematics, 22.10.2019 05:10

Geography, 22.10.2019 05:10


Mathematics, 22.10.2019 05:10

Biology, 22.10.2019 05:10

History, 22.10.2019 05:10


Mathematics, 22.10.2019 05:10

Mathematics, 22.10.2019 05:10

History, 22.10.2019 05:10

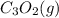 is -92.7 kJ/mol
is -92.7 kJ/mol![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0572/6547/72c39.png)
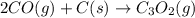
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(C_3O_2(g))})]-[(2\times \Delta H^o_f_{(CO(g))})+(1\times \Delta H^o_f_{(C(s))})]](/tpl/images/0572/6547/3be78.png)
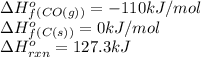
![127.3=[(1\times \Delta H^o_f_{(C_3O_2(g))})]-[(2\times (-110))+(1\times (0))]\\\\\Delta H^o_f_{(C_3O_2(g))}=-92.7kJ/mol](/tpl/images/0572/6547/5dacc.png)


