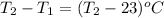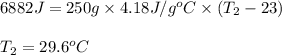The ΔH for the solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 6.21-g sample of NaOH dissolves in 250.0 g of water in a coffee-cup calorimeter, the temperature increases from 23.0 °C to °C. Assume that the solution has the same specific heat as liquid water, i. e., 4.18 J/g-K.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3


Chemistry, 23.06.2019 09:00, rebeccathecatt
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1
You know the right answer?
The ΔH for the solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When...
Questions in other subjects:



Mathematics, 16.10.2020 09:01





Mathematics, 16.10.2020 09:01

Biology, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01

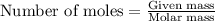
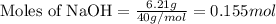
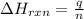
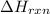 = enthalpy change of the reaction = 44.4 kJ/mol = 44400 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy change of the reaction = 44.4 kJ/mol = 44400 J/mol (Conversion factor: 1 kJ = 1000 J)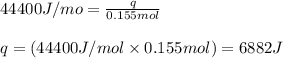
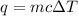
 = change in temperature =
= change in temperature =