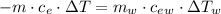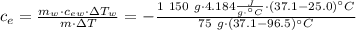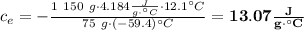Chemistry, 22.09.2019 00:30 Arianahinton9856
Achemist mixes 75.0 g of an unknown substance at 96.5°c with 1,150 g of water at 25.0°c. if the final temperature of the system is 37.1°c, what is the specific heat capacity of the substance? use 4.184 j / g °c for the specific heat capacity of water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, tristen2001
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3


Chemistry, 21.06.2019 17:30, hannahkelly3618
How many moles are equivalent to 55.5g of nano3
Answers: 1

Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1
You know the right answer?
Achemist mixes 75.0 g of an unknown substance at 96.5°c with 1,150 g of water at 25.0°c. if the fina...
Questions in other subjects:

Biology, 13.03.2020 00:00

History, 13.03.2020 00:00


Mathematics, 13.03.2020 00:00


Mathematics, 13.03.2020 00:00

Mathematics, 13.03.2020 00:00

World Languages, 13.03.2020 00:00

English, 13.03.2020 00:00

Mathematics, 13.03.2020 00:00