
Chemistry, 31.03.2020 00:57 jettskii214
9. Calculate the pH for each of the following cases in the titration of 35.0 mL of 0.200-M methylamine, CH3NH2(aq), with 0.200 M HCl(aq): (a) before addition of any HCl(aq) (b) after addition of 17.5 mL of HCl(aq) (c) after addition of 34.9 mL of HCl(aq) (d) after addition of 35.0 mL of HCl(aq) (e) after addition of 35.1 mL of HCl(aq)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, paulethjara
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
9. Calculate the pH for each of the following cases in the titration of 35.0 mL of 0.200-M methylami...
Questions in other subjects:




Mathematics, 09.07.2019 12:00


Biology, 09.07.2019 12:00




Mathematics, 09.07.2019 12:00

![K_{b} =\frac{[CH_{3}NH_{3}]+ [OH-] }{[CH_{3}NH_{2} ] }\\4.6x10^{-4} =\frac{x*x}{0.2-x} \\x=0.00936 M](/tpl/images/0572/2081/59e94.png)
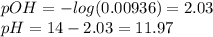
![[acid]=\frac{M_{2}V_{2} }{V_{1}+V_{2} } =\frac{0.2*17.5}{35+17.5} =0.0667M\\[base]=\frac{M_{1}V_{1} }{V_{1}+V_{2} } =\frac{0.2*35}{35+17.5} =0.13M](/tpl/images/0572/2081/75f14.png)
![pOH=pK_{b} +log(\frac{[salt]}{[base]} )=3.34+log(0.0667/0.066)=3.34\\pH=14-3.34=10.66](/tpl/images/0572/2081/0a576.png)
![[acid]=\frac{0.2*34.9}{35+34.9} =0.0998M\\\\ [base]=\frac{0.2*35}{35+34.9} =0.1M\\base-left=0.1-0.0998=0.0002M\\pOH=3.34+log(0.0998/0.0002)=6.04\\pH=14-6.04=7.96](/tpl/images/0572/2081/d31c6.png)
![[acid]=\frac{0.2*35}{35+35} =0.1M\\[base]=\frac{0.2*35}{35+35} =0.1M\\\\pH=7-\frac{1}{2} (pK_{b}+ log(c))=7-\frac{1}{2}(3.34+log(0.1))=5.83 \\](/tpl/images/0572/2081/b3303.png)
![[acid]=\frac{0.2*35.1}{35+35.1} =0.1M\\[base]=\frac{0.2*35}{35+35.1} =0.0998M\\acid-left=0.1-0.0998=0.0002M\\pH=-log(0.0002)=3.7\\](/tpl/images/0572/2081/1cd67.png)


