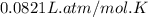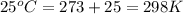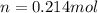Chemistry, 31.03.2020 00:57 MajentaSnow66
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2(s) + 2H2O(l)C2H2(g) + Ca(OH)2(aq) The product gas, C2H2, is collected over water at a temperature of 25 °C and a pressure of 748 mm Hg. If the wet C2H2 gas formed occupies a volume of 5.50 L, the number of moles of CaC2 reacted was mol. The vapor pressure of water is 23.8 mm Hg at 25 °C.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2...
Questions in other subjects:


History, 27.12.2019 17:31


Mathematics, 27.12.2019 17:31

Biology, 27.12.2019 17:31


Mathematics, 27.12.2019 17:31

Social Studies, 27.12.2019 17:31

Biology, 27.12.2019 17:31

Mathematics, 27.12.2019 17:31

 reacted was, 0.214 moles.
reacted was, 0.214 moles. gas.
gas.