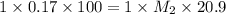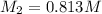Chemistry, 31.03.2020 01:05 bjpvrpow74wq
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3.56 grams of potassium hydrogen phthalate (KHP) into 100 mL of H2O. You then titrate the KHP with your sodium hydroxide solution and reach the endpoint after adding 20.9 mL of NaOH. What is the molarity of your sodium hydroxide solution? (Molecular Mass of KHP = 204.22 g/mol)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1


You know the right answer?
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3....
Questions in other subjects:

Biology, 20.07.2020 22:01

Mathematics, 20.07.2020 22:01

Chemistry, 20.07.2020 22:01

Mathematics, 20.07.2020 22:01



Mathematics, 20.07.2020 22:01

Mathematics, 20.07.2020 22:01


Mathematics, 20.07.2020 22:01

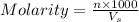
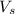 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml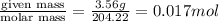
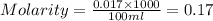
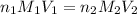
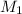 = molarity of KHP solution = 0.17 M
= molarity of KHP solution = 0.17 M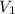 = volume of KHP solution = 100 ml
= volume of KHP solution = 100 ml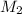 = molarity of NaOH solution = ?
= molarity of NaOH solution = ?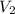 = volume of NaOH solution = 20.9 ml
= volume of NaOH solution = 20.9 ml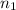 = valency of KHP = 1
= valency of KHP = 1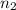 = valency of NaOH = 1
= valency of NaOH = 1