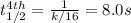Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
The reaction 3NO ---> N2O + NO2 is found to obey the rate law Rate = k[NO]^2^. If the first half-...
Questions in other subjects:

Mathematics, 10.05.2021 21:30

Chemistry, 10.05.2021 21:30

Computers and Technology, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30



![k=\frac{1}{[NO]_0*t_{/2}} = \frac{1}{[NO]_0*2.0}=\frac{1}{2[NO]_0}](/tpl/images/0572/1734/0a0ea.png)