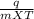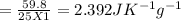Chemistry, 31.03.2020 00:53 amanda2517
You did an experiment in which you found that 59.8 J was required to raise the temperature of 25.0 g of ethylene glycol ( a compound used as antifreeze in automobile engines) by 1.00 Kelvin. Calculate the specific heat capacity of ethylene glycol from these data

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:30, creepycrepes
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
You did an experiment in which you found that 59.8 J was required to raise the temperature of 25.0 g...
Questions in other subjects:



Mathematics, 22.03.2020 03:04


Mathematics, 22.03.2020 03:05

Mathematics, 22.03.2020 03:05

Mathematics, 22.03.2020 03:05

Chemistry, 22.03.2020 03:05


Mathematics, 22.03.2020 03:05