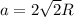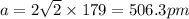Chemistry, 31.03.2020 00:23 tartcandi303
A certain metal M crystallizes in a lattice described by a face-centered cubic (fcc) unit cell. The radius r of M atoms has been measured to be 179.pm. Calculate the lattice constant a of a crystal of M. Be sure your answer has the correct number of significant digits, and be sure it has the correct unit symbol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


You know the right answer?
A certain metal M crystallizes in a lattice described by a face-centered cubic (fcc) unit cell. The...
Questions in other subjects:

Mathematics, 08.12.2020 19:40




Biology, 08.12.2020 19:40



Mathematics, 08.12.2020 19:40

German, 08.12.2020 19:40