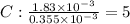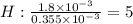Chemistry, 31.03.2020 00:27 CatsandDogsaredabest
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combustion of 29.5 mg produced 80.1 mg of CO2 and 16.4 mg of H2O. The molar mass of the compound was 162 g/mol. Determine its empirical and molecular formulas.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, Bryanguzman2004
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3


You know the right answer?
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combu...
Questions in other subjects:



Mathematics, 29.01.2020 04:11


Mathematics, 29.01.2020 04:11


Biology, 29.01.2020 04:11

Social Studies, 29.01.2020 04:11


Mathematics, 29.01.2020 04:11

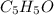 .
.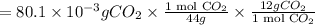
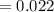 g
g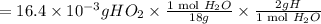
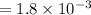 g
g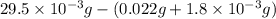
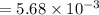 g
g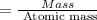
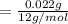
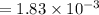 mol
mol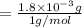
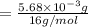
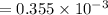 mol
mol