Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
You know the right answer?
How many grams of CO2 are produced by the combustion of 289 g of a mixture that is 28.6% CH4 and 71....
Questions in other subjects:

Mathematics, 18.09.2019 03:40


Chemistry, 18.09.2019 03:40


English, 18.09.2019 03:40





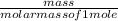
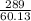
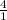 =
=