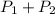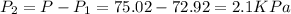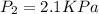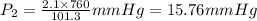Chemistry, 31.03.2020 00:16 rwbrayan8727
A gas is collected by water displacement so that its partial pressure is 72.92 kPa. The total pressure of the gas over water is 75.02 kPa. What is the temperature at which the gas was collected?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
A gas is collected by water displacement so that its partial pressure is 72.92 kPa. The total pressu...
Questions in other subjects:


English, 19.06.2021 05:30

Mathematics, 19.06.2021 05:30

Health, 19.06.2021 05:30

Mathematics, 19.06.2021 05:30


Social Studies, 19.06.2021 05:30

Mathematics, 19.06.2021 05:30


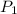 =72.92 KPa
=72.92 KPa