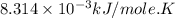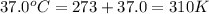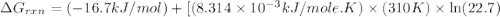Chemistry, 31.03.2020 00:01 samueltaye
Calculate the free energy change if the ratio of the concentrations of the products to the concentrations of the reactants is 22.7 and the temperature is 37.0 ° C ? Δ G ° ' for the reaction is − 16.7 kJ/mol .

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

You know the right answer?
Calculate the free energy change if the ratio of the concentrations of the products to the concentra...
Questions in other subjects:


Mathematics, 04.11.2020 22:30

Computers and Technology, 04.11.2020 22:30




Mathematics, 04.11.2020 22:30

Mathematics, 04.11.2020 22:30

Mathematics, 04.11.2020 22:30

Mathematics, 04.11.2020 22:30

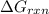 is -8.65 kJ/mol
is -8.65 kJ/mol ............(1)
............(1)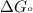 = standard Gibbs free energy = -16.7 kJ/mol
= standard Gibbs free energy = -16.7 kJ/mol