
Chemistry, 30.03.2020 23:51 kayyaybruh
For the reaction IO3–(aq) + 5 I–(aq) + 6 H+(aq) → 3 I2(aq) + 3 H2O(l) the rate of disappearance of I–(aq) at a particular time and concentration is 2.4 × 10–3 mol/L · s. What is the rate of appearance of I2(aq)?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
For the reaction IO3–(aq) + 5 I–(aq) + 6 H+(aq) → 3 I2(aq) + 3 H2O(l) the rate of disappearance of I...
Questions in other subjects:

Mathematics, 03.02.2020 22:46


Mathematics, 03.02.2020 22:46

Health, 03.02.2020 22:46






Mathematics, 03.02.2020 22:46

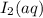 is
is 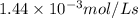
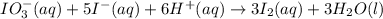
![Rate=-\frac{1d[I^-]}{5dt}=+\frac{d[I_2]}{3dt}](/tpl/images/0571/9488/09ead.png)
![-\frac{d[I^-]}{dt}]](/tpl/images/0571/9488/5d954.png) =
= 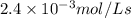
![+\frac{d[I_2]}{dt}=-\frac{3d[I^-]}{5dt}=-\frac{3}{5}\times 2.4\times 10^{-3}mol/Ls=1.44\times 10^{-3}mol/Ls](/tpl/images/0571/9488/1041e.png)


