
Chemistry, 30.03.2020 23:51 haileysolis5
If the reaction is carried out in a 22.4 L container, which initial amounts of X and Y will result in the formation of solid XY? If the reaction is carried out in a 22.4 container, which initial amounts of and will result in the formation of solid ? 2.0 mol X; 2.0 molY 6 mol X; 0.6 molY 1 mol X; 1 molY none of the above

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
If the reaction is carried out in a 22.4 L container, which initial amounts of X and Y will result i...
Questions in other subjects:

Mathematics, 06.05.2021 18:30



Mathematics, 06.05.2021 18:30



Mathematics, 06.05.2021 18:30


Chemistry, 06.05.2021 18:30

Mathematics, 06.05.2021 18:30

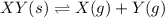 Kp = 4.1 (at 0°C)
Kp = 4.1 (at 0°C)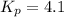
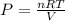
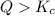 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.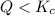 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.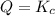 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

