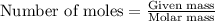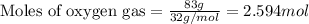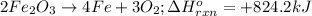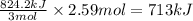Consider the reaction:
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of...

Chemistry, 30.03.2020 23:14 constipatedcow18
Consider the reaction:
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of O2 results in:
A. the absorption of 22800 kJ of heat.
B. the release of 2140 kJ of heat.
C. the absorption of 2140 kJ of heat.
D. the release of 713 kJ of heat.
E. the absorption of 713 kJ of heat.
F. the release of 22800 kJ of heat.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Questions in other subjects:




Law, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00



Geography, 12.12.2020 17:00