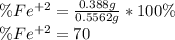Chemistry, 30.03.2020 23:08 GreenHerbz206
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solution. This solution is then titrated with 28.72 mL of 0.04021 M K2Cr2O7 (aq). What is the percent by mass iron in the ore sample

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:40, raquelqueengucci25
In this synthesis reaction what products will form
Answers: 1

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solut...
Questions in other subjects:






Physics, 14.09.2019 09:10




Computers and Technology, 14.09.2019 09:10