
Chemistry, 30.03.2020 23:11 pedroramirezr2
130.0 grams of NaNO 3 is dissolved in water to make a 5.00 L solution. What is the molarity of the solution? (Molar mass of NaNo3 =g/mol) what is the molarity

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
130.0 grams of NaNO 3 is dissolved in water to make a 5.00 L solution. What is the molarity of the s...
Questions in other subjects:

Health, 23.04.2021 19:50



Health, 23.04.2021 19:50

History, 23.04.2021 19:50


History, 23.04.2021 19:50

Biology, 23.04.2021 19:50



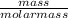 =
= 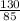 = 1.53moles
= 1.53moles  = 0.31M
= 0.31M

