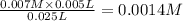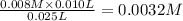A student working in the laboratory prepared the following reactants: 5 mL of 0.007M Cd2+(aq) 10 mL of 0.008M SCN-(aq) 10 mL of 0.5M HNO3(aq) These reagents were mixed and allowed to stand for 10 minutes. The concentration of Cd(SCN)+ in the resulting equilibrium mixture is found to be 5 x 10−4M. Calculate the initial concentration of Cd2+(aq).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
A student working in the laboratory prepared the following reactants: 5 mL of 0.007M Cd2+(aq) 10 mL...
Questions in other subjects:


Mathematics, 05.12.2019 07:31







Mathematics, 05.12.2019 07:31

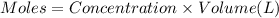
![[Cd^{2+}]=0.007M](/tpl/images/0571/8149/5e85a.png)
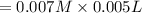
![[SCN^{-}]=0.008 M](/tpl/images/0571/8149/33083.png)
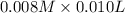
![[HNO_3]=0.5 M](/tpl/images/0571/8149/6e078.png)