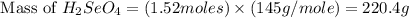Chemistry, 30.03.2020 22:32 savannahvargas512
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O. Calculate the maximum mass (in grams) of selenic acid, H2SeO4, that can be produced in the reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 12:00, angieplasencia8
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O....
Questions in other subjects:

History, 26.01.2020 20:31


Advanced Placement (AP), 26.01.2020 20:31


Mathematics, 26.01.2020 20:31


Mathematics, 26.01.2020 20:31



Mathematics, 26.01.2020 20:31


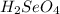 produced is, 220.4 grams.
produced is, 220.4 grams. = 431 g
= 431 g

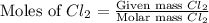
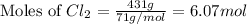


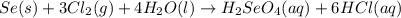
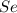
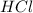 react with
react with  moles of
moles of 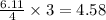 moles of
moles of 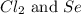 are excess reagent because the given moles are greater than the required moles and
are excess reagent because the given moles are greater than the required moles and