
Chemistry, 30.03.2020 22:35 tyrareed702
Roasting galena [lead(II) sulfide] is an early step in the industrial isolation of lead. How many liters of sulfur dioxide, measured at STP, are produced by the reaction of 3.73 kg of galena with 170. L of oxygen gas at 220°C and 2.00 atm? Lead(II) oxide also forms.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 05:00, jayden6467
How many moles are in 7.2 x 10^23 carbon molecules?
Answers: 1

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
Roasting galena [lead(II) sulfide] is an early step in the industrial isolation of lead. How many li...
Questions in other subjects:



Mathematics, 25.06.2019 05:30



History, 25.06.2019 05:30


Mathematics, 25.06.2019 05:30


Mathematics, 25.06.2019 05:30

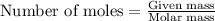
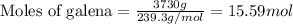
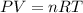
![220^oC=[220+273]K=493K](/tpl/images/0571/5966/b1a8e.png)
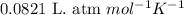
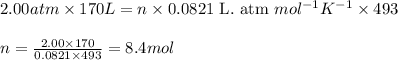
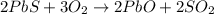
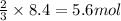 of galena
of galena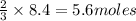 of sulfur dioxide
of sulfur dioxide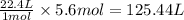 of volume
of volume

