
Chemistry, 30.03.2020 21:43 sindy35111
1. Based on the appearance of your reaction in the beaker, which reagent do you think was consumed and which reagent had some left over? The aluminum was consumed, and copper was left over as seen by the reddish particles. 2. If 5.0 g of iron metal is reacted with 15.0 g of Cl2 gas, how many grams of ferric chloride will form? About 14.52 grams will form. 3. For the reaction above the amount of ferric chloride obtained in the lab was 9.15 grams. Calculate the percent yield. The percent yield would be around 63.02%. 4. What are some reasons for obtaining a percent yield of less than 100 percent? Factors such as the reactants not reacting completely, human error in the experiment, the reactants might have too large of a surface area for reaction, multiple reactions occurring within an experiment, temperature, etc.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1



Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
1. Based on the appearance of your reaction in the beaker, which reagent do you think was consumed a...
Questions in other subjects:







Mathematics, 06.05.2020 20:30

Mathematics, 06.05.2020 20:30

Biology, 06.05.2020 20:30

Mathematics, 06.05.2020 20:30

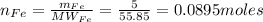
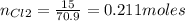
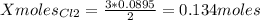
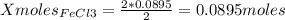
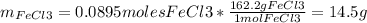
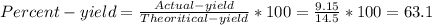 %
%

