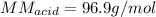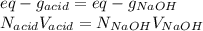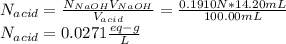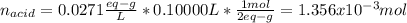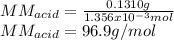Chemistry, 30.03.2020 21:27 saadizak7098
6. A 0.1310 g sample of an unknown diprotic acid is diluted to 100.00 mL and titrated by using 0.1910 M NaOH. If 14.20 mL of the NaOH solution is required to reach the second equivalence point, what is the molar mass of the acid?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
6. A 0.1310 g sample of an unknown diprotic acid is diluted to 100.00 mL and titrated by using 0.191...
Questions in other subjects:

Advanced Placement (AP), 29.09.2021 23:30




Mathematics, 29.09.2021 23:30



Mathematics, 29.09.2021 23:30

English, 29.09.2021 23:30