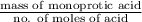Chemistry, 30.03.2020 21:31 anggelkevin100
A 0.410 g sample of a monoprotic acid is dissolved in water and titrated with 0.190 M KOH . What is the molar mass of the acid if 24.0 mL of the KOH solution is required to neutralize the sample?

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
A 0.410 g sample of a monoprotic acid is dissolved in water and titrated with 0.190 M KOH . What is...
Questions in other subjects:


Mathematics, 09.11.2019 08:31



Health, 09.11.2019 08:31

Mathematics, 09.11.2019 08:31


Social Studies, 09.11.2019 08:31

Social Studies, 09.11.2019 08:31