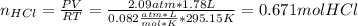Chemistry, 30.03.2020 21:05 jailenevazquez755
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 mL of water to form hydrochloric acid solution. Calculate the molarity of the solution. Assume no change in volume.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, Brittpaulina
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 m...
Questions in other subjects: