
Chemistry, 30.03.2020 21:06 annagwhiteou0hrh
Calculate the standard entropy change for the reaction: 2 hydrogen sulfide(g) plus sulfur dioxide (g) goes to form 3 sulfur(s) plus 2 water(g). Given S(s) = 31.88 J/mol. Look up the other values in the text appendix. No units required. Just the numerical answer

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Calculate the standard entropy change for the reaction: 2 hydrogen sulfide(g) plus sulfur dioxide (g...
Questions in other subjects:

Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

English, 14.01.2021 01:00

English, 14.01.2021 01:00

Arts, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00


English, 14.01.2021 01:00

![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0571/2033/52737.png)
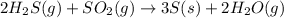
![\Delta S^o_{rxn}=[(3\times \Delta S^o_{(S(s))})+(2\times \Delta S^o_{(H_2O(g))})]-[(2\times \Delta S^o_{(H_2S(g))})+(1\times \Delta S^o_{(SO_2(g))})]](/tpl/images/0571/2033/492b6.png)
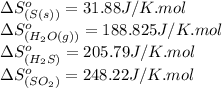
![\Delta S^o_{rxn}=[(3\times (31.88))+(2\times (188.825))]-[(2\times (205.79))+(1\times (248.22))]\\\\\Delta S^o_{rxn}=-186.51J/K](/tpl/images/0571/2033/7cc5d.png)



