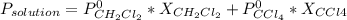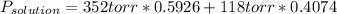Chemistry, 30.03.2020 21:07 villarrealc1987
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) among the many cancer causing volatile chlorinated organic compounds. An autoshop you are employed at keeps a jar (closed) of this solution to use as a solvent for cleaning parts. If the jar contains 1.60mol of dichloromethane and 1.10mol of CCl4, what would be the total pressure in the jar if the shop is kept at a consistent 23.5C

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, braydentillery1221
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) a...
Questions in other subjects:







History, 05.05.2020 03:37


English, 05.05.2020 03:37