
Chemistry, 30.03.2020 21:10 Franklyn3220
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enough water to make a final volume of 75.0 mLmL. Part A Assuming that all of the solid dissolves, what is the pH of the final solution

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 04:00, josephicarusmarrujo
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enoug...
Questions in other subjects:


Mathematics, 29.09.2020 08:01

Mathematics, 29.09.2020 08:01

Mathematics, 29.09.2020 08:01

Mathematics, 29.09.2020 08:01


Mathematics, 29.09.2020 08:01

Mathematics, 29.09.2020 08:01

Mathematics, 29.09.2020 08:01

 =
=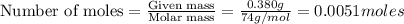
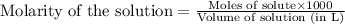
 solution = 1.45 M
solution = 1.45 M
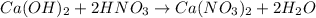
 moles of
moles of  are left in 75.0 ml of solution
are left in 75.0 ml of solution
![pH=-\log [H^+]](/tpl/images/0571/2204/37e81.png)
![pH=-\log[0.83]](/tpl/images/0571/2204/f1115.png)



