
Chemistry, 30.03.2020 20:46 joannegrace869
Problem PageQuestion Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. Calculate the moles of acetylene needed to produce 2.00 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, blondieb1722
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Problem PageQuestion Acetylene gas is often used in welding torches because of the very high heat pr...
Questions in other subjects:






Mathematics, 07.01.2021 06:50

Mathematics, 07.01.2021 06:50


History, 07.01.2021 06:50

Mathematics, 07.01.2021 06:50

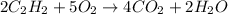
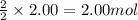 of acetylene gas
of acetylene gas

