Chemistry, 30.03.2020 20:35 esanchez2002fcb
Dry ice is solid carbon dioxide. A 1.65−g sample of dry ice is placed in an evacuated 3.93−L vessel at 23.0°C. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Dry ice is solid carbon dioxide. A 1.65−g sample of dry ice is placed in an evacuated 3.93−L vessel...
Questions in other subjects:


Mathematics, 20.05.2021 15:50



Arts, 20.05.2021 15:50

History, 20.05.2021 15:50



English, 20.05.2021 15:50

Mathematics, 20.05.2021 15:50

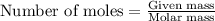
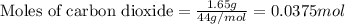
![23^oC=[23+273]=296K](/tpl/images/0571/0457/eb892.png)