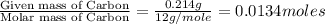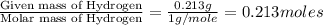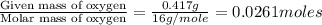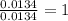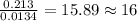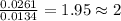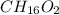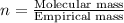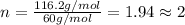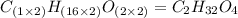Chemistry, 30.03.2020 21:13 robertjoy19
The smell of dirty gym socks is caused by the compound caproic acid(contains H, C, O). Combustion of 0.844 g of caproic acid produced 0.784 g of H2O and 1.92 g of CO2. If the molar mass of caproic acid is 116.2 g/mol, what is the molecular formula of caproic acid? (MM C = 12g/mol, H = 1g/mol, O = 16 g/mol)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
The smell of dirty gym socks is caused by the compound caproic acid(contains H, C, O). Combustion of...
Questions in other subjects:

Computers and Technology, 17.10.2019 04:40


Biology, 17.10.2019 04:40



Arts, 17.10.2019 04:40

Biology, 17.10.2019 04:40

Mathematics, 17.10.2019 04:40

English, 17.10.2019 04:40

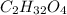
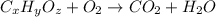
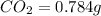
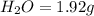
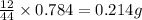 of carbon will be contained.
of carbon will be contained.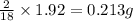 of hydrogen will be contained.
of hydrogen will be contained.