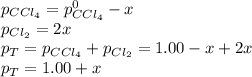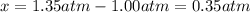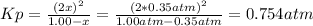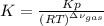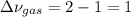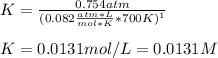Chemistry, 30.03.2020 20:36 Anaaguayo954
At elevated temperature, carbon tetrachloride decomposes to its elements: CCl4(g) C(s) 2Cl2(g). At 700 K, if the initial pressure of CCl4 is 1. 00 atm and at equilibrium the total pressure is 1. 35 atm, then calculate K

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2


Chemistry, 23.06.2019 07:50, alexusnicole817
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
At elevated temperature, carbon tetrachloride decomposes to its elements: CCl4(g) C(s) 2Cl2(g). At 7...
Questions in other subjects:


Mathematics, 21.01.2021 01:40


History, 21.01.2021 01:40

Computers and Technology, 21.01.2021 01:40


Mathematics, 21.01.2021 01:40

Mathematics, 21.01.2021 01:40


Mathematics, 21.01.2021 01:40

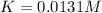
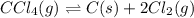
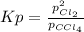
 due to reaction extent:
due to reaction extent: