
Chemistry, 30.03.2020 20:27 Lovelybunny321
Instead of using ratios for back titrations we can also use molarities, if our solutions are standardized. A 0.196 g sample of antacid containing an unknown amount of triprotic base Al(OH)3 was reacted with 25.0mL of 0.111M HCl. The resulting solution was then titrated with 11.05mL of 0.132M NaOH solution. Calculate the mass percent of Al(OH)3 in the antacid sample.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 21.06.2019 22:30, stefaniethibodeaux
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 01:50, lildestinyquintana
Ase your answer to this question on the information below. hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant. the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen. chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product. nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1
You know the right answer?
Instead of using ratios for back titrations we can also use molarities, if our solutions are standar...
Questions in other subjects:

Physics, 16.02.2021 21:20



Mathematics, 16.02.2021 21:20


Mathematics, 16.02.2021 21:20


Mathematics, 16.02.2021 21:20

Computers and Technology, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20

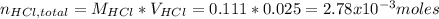
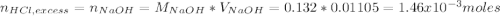
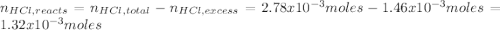
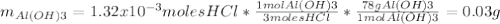
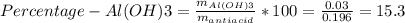 %
%

