
Chemistry, 30.03.2020 21:38 zhuotingwu147
For the aqueous reaction dihydroxyacetone phosphate is the reactant and glyceraldehyde 3 phosphate is the product. dihydroxyacetone phosphate − ⇀ ↽ − glyceraldehyde − 3 − phosphate the standard change in Gibbs free energy is Δ G ° ' = 7.53 kJ/mol . Calculate Δ G for this reaction at 298 K when [dihydroxyacetone phosphate] = 0.100 M and [glyceraldehyde-3-phosphate] = 0.00400 M . Δ G = kJ / mol

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 05:50, kawaunmartinjr10
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1
You know the right answer?
For the aqueous reaction dihydroxyacetone phosphate is the reactant and glyceraldehyde 3 phosphate i...
Questions in other subjects:

Chemistry, 28.05.2021 20:20


Mathematics, 28.05.2021 20:20

Social Studies, 28.05.2021 20:20







 of above equation is:
of above equation is:![K_{eq}=\frac{\text{[Glyceraldehyde-3-phosphate]}}{\text{[Dihydroxyacetone phosphate]}}](/tpl/images/0571/3632/f265a.png)


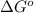 = Standard Gibbs free energy = 7.53 kJ/mol = 7530 J/mol
= Standard Gibbs free energy = 7.53 kJ/mol = 7530 J/mol



