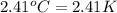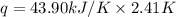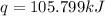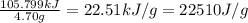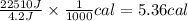A researcher studying the nutritional value of a new candy places a 4.70 g 4.70 g sample of the candy inside a bomb calorimeter and combusts it in excess oxygen. The observed temperature increase is 2.41 ∘ C. 2.41 ∘C. If the heat capacity of the calorimeter is 43.90 kJ ⋅ K − 1 , 43.90 kJ⋅K−1, how many nutritional Calories are there per gram of the candy?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
A researcher studying the nutritional value of a new candy places a 4.70 g 4.70 g sample of the cand...
Questions in other subjects:

Mathematics, 16.12.2021 01:00


History, 16.12.2021 01:00

Mathematics, 16.12.2021 01:00

Mathematics, 16.12.2021 01:00

Mathematics, 16.12.2021 01:00



English, 16.12.2021 01:00

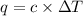
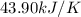
 = change in temperature =
= change in temperature =