
Chemistry, 30.03.2020 20:16 Duckkkkkkkk
In each row check off the boxes that apply to the highlighted reactant. reaction The highlighted reactant acts as a... (check all that apply)
1. HCH3CO2(aq) + NH3(aq) → CH3CO−2(aq) + NH+4(aq)
A. Brønsted-Lowry acid
B. Brønsted-Lowry base
C. Lewis acid
D. Lewis base
2. BH3(aq) + NH3(aq) → BH3NH3(aq)
A. Brønsted-Lowry acid
B. Brønsted-Lowry base
C. Lewis acid
D. Lewis base
3. HNO2(aq) + C2H5NH2(aq) → NO−2(aq) + C2H5NH+3(aq)
A. Brønsted-Lowry acid
B. Brønsted-Lowry base
C. Lewis acid
D. Lewis base

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tiwaribianca475
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2


Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1
You know the right answer?
In each row check off the boxes that apply to the highlighted reactant. reaction The highlighted rea...
Questions in other subjects:


Mathematics, 22.09.2019 04:20



Mathematics, 22.09.2019 04:20


English, 22.09.2019 04:20


Mathematics, 22.09.2019 04:20

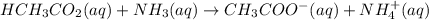
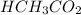
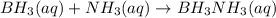
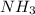

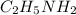

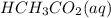 is donating a proton , it acts as a bronsted acid.
is donating a proton , it acts as a bronsted acid.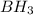 and act as lewi base.
and act as lewi base. is accepting a proton , it acts as a bronsted base.
is accepting a proton , it acts as a bronsted base.

