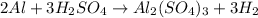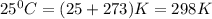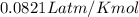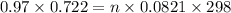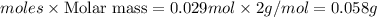G A sample that contains aluminum is reacted with sulfuric acid according to the equation given below. When the aluminum dissolved the total volume of gas collected over water at 25 °C is 0.722 L at a total pressure of 739 mm Hg. What mass of hydrogen gas is collected?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
G A sample that contains aluminum is reacted with sulfuric acid according to the equation given belo...
Questions in other subjects:

Mathematics, 25.10.2021 19:00

English, 25.10.2021 19:00

Chemistry, 25.10.2021 19:00

English, 25.10.2021 19:00

Mathematics, 25.10.2021 19:00


Biology, 25.10.2021 19:00

History, 25.10.2021 19:00

Mathematics, 25.10.2021 19:00

Mathematics, 25.10.2021 19:00