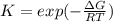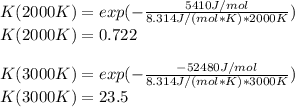The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X 2 ( g ) ⟶ X ( g ) Assume that the standard molar Gibbs energy of formation of X(g) is 5.41 kJ⋅mol − 1 at 2000. K and − 52.48 kJ⋅mol − 1 at 3000. K. Determine the value of K (the thermodynamic equilibrium constant) at each temperature. K at 2000. K = K at 3000. K =

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

You know the right answer?
The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X...
Questions in other subjects:


Computers and Technology, 01.03.2021 21:50


Mathematics, 01.03.2021 21:50

Advanced Placement (AP), 01.03.2021 21:50




History, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50