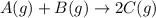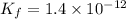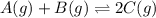Chemistry, 30.03.2020 19:28 starlightmoon213
Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-12 M-1s-1 for the reaction: A(g) + B(g) → 2C(g) at 25°C and k equals 2.7 × 10-13 M-1s-1 for the reaction: 2C(g) → A(g) + B(g) at 25°C.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-1...
Questions in other subjects:

History, 20.09.2019 09:00

Mathematics, 20.09.2019 09:00


English, 20.09.2019 09:00

Mathematics, 20.09.2019 09:00

Spanish, 20.09.2019 09:00




Business, 20.09.2019 09:00