

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, arnold2619
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
A solution containing CaCl2 is mixed with a solution of Li2C2O4 to form a solution that is 3.5 × 10-...
Questions in other subjects:

English, 10.02.2021 01:40

Mathematics, 10.02.2021 01:40





Mathematics, 10.02.2021 01:40

Mathematics, 10.02.2021 01:40

Social Studies, 10.02.2021 01:40

Chemistry, 10.02.2021 01:40

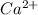 =
= 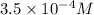
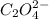 =
= 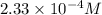
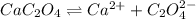
![K_{sp}=[Ca^{2+}][C_2O_4^{2-}]=2.3\times 10^{-9}](/tpl/images/0570/7450/df299.png)
![Q_{sp}=[Ca^{2+}][C_2O_4^{2-}]](/tpl/images/0570/7450/af00c.png)
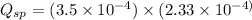
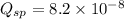
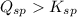 that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.
that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.

