
Chemistry, 30.03.2020 19:07 turtlesage21
G For the reaction N2(g) + O2(g) --> 2 NO(g), the equilibrium constant, K=1.0x10-5 Which direction will the reaction move, and why, if the reactants and products have the following concentrations: [N2]=0.015 M [O2]=0.25 M [NO]=0.010

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2


Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
G For the reaction N2(g) + O2(g) --> 2 NO(g), the equilibrium constant, K=1.0x10-5 Which directio...
Questions in other subjects:




History, 19.07.2019 16:00

Social Studies, 19.07.2019 16:00





![\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0570/7732/10229.png) where the terms in the large bracket denote the concentrations of respective reacting species.
where the terms in the large bracket denote the concentrations of respective reacting species.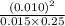 =
= 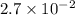
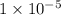 , we see that the Q is larger than that of K.
, we see that the Q is larger than that of K.


