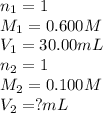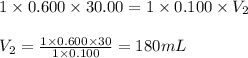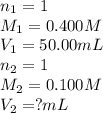Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1


Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
You know the right answer?
Determine the volume at the equivalence point if a 0.100 M NaOH(aq) solution is used to titrate the...
Questions in other subjects:

Mathematics, 20.05.2020 23:59

Mathematics, 21.05.2020 00:00



Mathematics, 21.05.2020 00:00

Geography, 21.05.2020 00:00


English, 21.05.2020 00:00

History, 21.05.2020 00:00

Mathematics, 21.05.2020 00:00

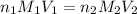
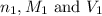 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid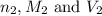 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base