
A sealed isothermal container initially contains 4 moles of methanol gas, CH3OH. The following reversible reaction occurs: CH3OH (g) D 2 H2 (g) +CO (g) At equilibrium, there were 3 moles of CH3OH in the container. What is the total number of moles of gas present in the container at equilibrium?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2


Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
A sealed isothermal container initially contains 4 moles of methanol gas, CH3OH. The following rever...
Questions in other subjects:


Mathematics, 10.04.2020 18:58








Physics, 10.04.2020 18:58

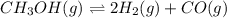
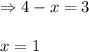
![[n_{CH_3OH}+n_{H_2}+n_{CO}]=[3+2+1]=6mol](/tpl/images/0570/7796/f27cd.png)


