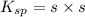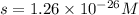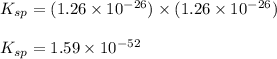Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, pleasehelpmeonthis
The difference between atomic mass and molar mass
Answers: 3


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
At 25 oC the solubility of mercury(II) sulfide is 1.26 x 10-26 mol/L. Calculate the value of Ksp at...
Questions in other subjects:

German, 12.06.2021 02:20

Social Studies, 12.06.2021 02:20

Mathematics, 12.06.2021 02:20



Mathematics, 12.06.2021 02:20




Mathematics, 12.06.2021 02:20

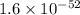
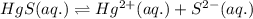
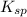 for above equation follows:
for above equation follows: