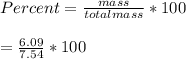Chemistry, 30.03.2020 17:42 haileesprague3999
Chalk is a mixture of CaCO3 and CaSO4. When added to HCl, only the CaCO3 reacts according to the reaction:
CaCO3(s) + 2 HCl (aq) → CaCl2 (aq) + CO2(g) + H2O (l)
When a 7.54 g piece of chalk is placed in HCl, 2.68 of CO2 is produced. What percentage of the chalk is CaCO3?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2


Chemistry, 23.06.2019 04:31, laurenbreellamerritt
How big are the bighest ocean waves at mavericks
Answers: 1
You know the right answer?
Chalk is a mixture of CaCO3 and CaSO4. When added to HCl, only the CaCO3 reacts according to the rea...
Questions in other subjects:


Social Studies, 31.08.2020 22:01

Computers and Technology, 31.08.2020 22:01


Mathematics, 31.08.2020 22:01

Biology, 31.08.2020 22:01



Computers and Technology, 31.08.2020 22:01