Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
In a liquid mixture of chloroform (C) and acetone (A) with chloroform mole fraction xC = 0.4693, the...
Questions in other subjects:


Mathematics, 07.01.2021 01:20


Mathematics, 07.01.2021 01:20

Mathematics, 07.01.2021 01:20

Spanish, 07.01.2021 01:20

History, 07.01.2021 01:20

Mathematics, 07.01.2021 01:20

Mathematics, 07.01.2021 01:20

Mathematics, 07.01.2021 01:20

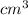
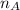
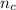
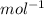
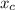 ) × 58.08 + n
) × 58.08 + n 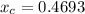
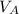 +
+