
Chemistry, 30.03.2020 16:55 jc95704816
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assume that all the radiation is absorbed and converted to heat. How many photons are required for the liquid to absorb 58.28 J 58.28 J of heat?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

You know the right answer?
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assum...
Questions in other subjects:





Mathematics, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

History, 05.11.2019 08:31


Mathematics, 05.11.2019 08:31

History, 05.11.2019 08:31

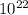 Photons
Photons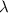 = 6.92 ×
= 6.92 × 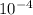 cm = 6.92 ×
cm = 6.92 ×  m
m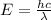
 J - s
J - s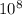
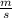
 ×
× 
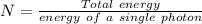
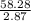 ×
× 

