
Chemistry, 30.03.2020 17:02 Diamondnado3046
You mix 200. mL of 0.400M HCl with 200. mL of 0.400M NaOH in a coffee cup calorimeter. The temperature of the solution goes from 25.10°C to 27.78° C. What is the molar enthalpy of neutralization of the acid in kJ/mol? Assume all densities are 1.00 g/mL and the specific heat capacities are 4.184 J/g*K.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1


Chemistry, 23.06.2019 00:30, kylee65
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
You mix 200. mL of 0.400M HCl with 200. mL of 0.400M NaOH in a coffee cup calorimeter. The temperatu...
Questions in other subjects:

Mathematics, 03.08.2019 10:00


Mathematics, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00


Physics, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00



Mathematics, 03.08.2019 10:00






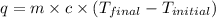
 = specific heat of water =
= specific heat of water = 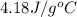
 = final temperature of water =
= final temperature of water = 
 = initial temperature of metal =
= initial temperature of metal = 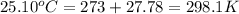


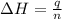
 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?


